Animal model and blood sampling
All animals were kept under specific pathogen-free conditions in the Inserm-UMS44 animal facility of the Paul Brusse Hospital, Villejuif, France. The health status of the mice was regularly monitored by the animal facility staff. A local temperature of 23-25°C, humidity of 40-60% and a 12/12 h light/dark cycle were observed. Mice were kept in individually ventilated cages with 3-5 animals and had ample access to regular food and water. All necessary conditions have been validated according to the ethical committee CAPSud—Comité d’éthique CEEA n°26.
To collect normal mouse blood, 8-14 week old female wild type C57BL/6 mice (30 in total) (Envigo, Gana, France) were used. Sterile glass microcapillary tubes (Fisher Scientific, Illkirch, France) were first washed with PBS-EDTA solution (final concentration 1.8 mg/ml). Then, immediately after sacrificing the cervically dislocated mice, the maximum amount of blood was collected from each mouse sinus by cardiac puncture or retro-orbital to reach the final required blood volume. All animal experimental procedures throughout this project were verified by the animal welfare structure of the animal facility (la structure du bien être animal (SBEA)) and were carried out under appropriate ethical and animal welfare practices.
PyMY transgenic mice were maintained locally under standard conditions as previously described. Upon the appearance of mammary tumors, mice (5 in total) were anesthetized with isoflurane gas, sacrificed by cervical dislocation, and approximately 1 ml of blood was collected by cardiac puncture. Immediately after collection, blood was transferred into heparin (Sarstedt, Nümbrecht, Germany) or K2-EDTA vacutainers (Becton Dickinson, Mississauga, Canada).
Preparation of tumor cell lines
4T1 mouse breast cancer cell line (passages 12–15) and MCF7 human breast cancer cell line (passages 7–10) were provided by Inserm U1197 (Paul Brousse Hospital, Villejuif, France) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Life Technologies, USA) supplemented with 10% FBS (Pan biotech, Eidenbach, Germany) and 1% penicillin/streptomycin (P/S) (Thermofisher Scientific, Waltham, MA, USA). Upon reaching 80% confluency, cells were detached using trypsin-EDTA (Thermofisher Scientific, Waltham, MA, USA), counted manually using trypan blue, and diluted to the required number in PBS 1X (Thermofisher Scientific, Waltham, MA, USA). Cells were then added directly to collected mouse blood for further experimental procedures.
CTC Isolation using ScreenCell Cyto Kit
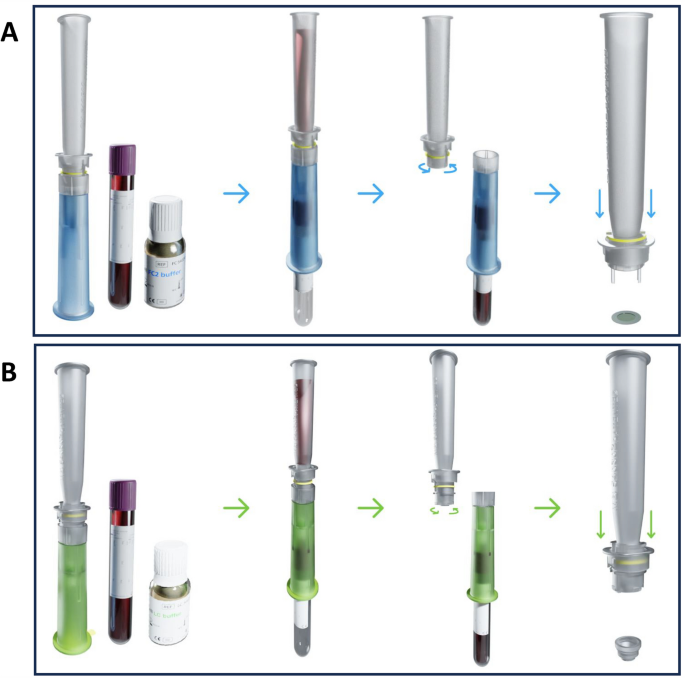
Single and cluster CTCs were isolated using a ScreenCell® Cyto device (ScreenCell, Paris, France). Briefly, different volumes of whole blood (50, 100, 200, 500, and 1000 µl) were diluted with PBS-EDTA solution to 3 mL. The diluted blood samples were then incubated with 4 ml of ScreenCell FC2 fixation buffer and incubated at room temperature for 8 min to lyse red blood cells (RBCs) and preserve nucleated cells. The diluted blood samples were then transferred to a ScreenCell Cyto device holding a separation support (IS) with an 18 µm thick polycarbonate membrane with randomly distributed circular pores (6.5 ± 0.33 µm). The diluted blood was then passed through the membrane by vacuum force, and 1.6 ml of PBS 1X (Thermofisher Scientific, Waltham, MA, USA) was added at the end of the filtration to remove the remaining blood waste. The IS were then removed from the ScreenCell Cyto device, rinsed with PBS 1X, dried on absorbent tissue, and stained with RAL555 (catalog no. 720-0351, VWR, International, Radnor, PA, USA) for cytomorphological analysis. Characterization and counting of CTCs was performed by an experienced cytopathologist (JW) blinded to the histological diagnosis using a NIKON eclipse 80i microscope integrated with a cooled CCD camera system and NIS-Elements BR2.30 imaging software (Nikon, Tokyo, Japan). Captured cells were classified as CTCs if they fulfilled all three cytological criteria: 1) nuclei >3x the calibrated 6.5 μm ScreenCell IS pore size, 2) irregular nuclear contour, and 3) high nuclear/cytoplasmic ratio.
CTC isolation using the ScreenCell MB kit
Different volumes of mouse blood samples (20, 50, 100, 200, 500 µl) were diluted with PBS-EDTA solution to 6 mL and incubated with 1 ml of ScreenCell LC dilution buffer for 2 min at room temperature. CTC enrichment was performed using the ScreenCell MB device. At the end of the enrichment step, blood waste was removed from the MB IS using 1.6 ml RNase-free PBS 1X (Thermofisher Scientific, Waltham, MA, USA) and transferred to a 1.5 ml microcentrifuge tube for further DNA extraction steps.
Screen Cell Technology Recovery Rate
To evaluate the recovery rate of the ScreenCell technology, 0, 5, or 10 cells were precisely harvested under a microscope and added to 100 µl blood samples from healthy WT mice. These blood samples were then processed using the ScreenCell Cyto kit (ScreenCell, Paris, France) as previously described. The IS were then stained with RAL555 (catalog no. 720-0351, VWR, International, Radnor, PA, USA) for cytomorphological analysis. CTC counting was performed using a NIKON eclipse 80i microscope (Nikon, Tokyo, Japan).
DNA extraction and quantification
Genomic DNA was isolated from cells captured with the MB Kit IS using the QIAamp DNA Micro Kit, Catalog No./ID: 56304 (Qiagen, Hilden, Germany). 105 µl of lysis buffer was added to the MB Kit IS. The IS was then incubated at 56 °C for 10 min and centrifuged at 12000 g for 1 min. DNA extraction was performed according to the manufacturer’s protocol. Genomic DNA was eluted in 25 µl of nuclease-free water (Thermo Fisher Scientific, Waltham, MA, USA).
DNA concentrations were measured using the Qubit 1X dsDNA HS Assay Kit (high sensitivity, 0.1–120 ng) and a Qubit 4.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. A sample volume of 1 μl was added to 199 μl of Qubit working solution.
CTC culture using the ScreenCell MB kit
100 µl of WT mouse blood sample containing 500 spiked 4T1 cancer cells was diluted to 6 ml with PBS-EDTA solution and then incubated with 1 ml LC dilution buffer for 2 min at room temperature. At the end of the incubation, the lysis procedure was stopped by adding 1.6 ml of DMEM medium containing 10% FBS and 1% P/S, and enrichment was performed using the ScreenCell MB device. IS were then released directly into 24-well tissue culture plates. CTCs were cultured for 10 days at 37 °C in a humidified atmosphere of 5% CO2 in DMEM supplemented with 10% FBS (Pan biotech, Eidenbach, Germany) and 1% P/S (Thermofisher Scientific, Waltham, MA, USA). The medium was changed every 2 days.